
Unique Device Identification (UDI) systems are being implemented globally. Whether it’s the USA’s FDA UDI, Europe’s EU MDR, India’s CDSCO, or China’s NMPA, countries from north to south and east to west are adopting UDI systems. UDI improves patient safety, enhances the traceability of medical devices, and streamlines regulatory processes.
To manage these evolving regulations globally, medical device organizations need to focus on a cloud-based approach to labeling and packaging and automate data management to ensure accurate labeling and UDI integrity.
In this whitepaper we look at how to navigate this complex journey from the start, how to circumnavigate UDI around the world with each region’s nuances, and how future proof cloud solution choices can set the right direction for medical device organizations to meet these labeling challenges.
Mislabeling continues to be a leading cause of medical device recalls, with manufacturers struggling to coordinate labeling data across their organizations. As an example, in 2022, nearly 900 medical devices were recalled by the FDA ¹. In addition, many products are mislabeled and caught prior to shipping into the market, requiring difficult label rework and corrective action, otherwise products must be discarded.
It indicates an alarming snapshot of the industry’s quality issues and a potential threat to public health. Soaring recalls lead to serious long-term reputational and financial damage for healthcare organizations and these errors affect all areas of the wider supply chain and create multiple challenges internally with product design, production, inspection, compliance, and other functions.
To help manage this, many life science manufacturers turn to Product Lifecycle Management (PLM) systems, but these solutions don’t sufficiently address the myriad of design and data management complexities across domains. Instead, PLM focuses on engineering disciplines like mechanical, electrical, software development, and the overall digitalization of design control.
Until labeling becomes a real issue, it’s often both overlooked and underappreciated as companies take an “if it’s not broken, don’t fix it” mindset to their labeling and packaging operations. And even when attention is focused on labeling, the new tools are selected for different specializations rather than to address broader challenges and their impact across the supply chain.
Precision is paramount in healthcare, where one inaccurate piece of data, misinterpretation, or misdiagnosis can have profound consequences on patient care. Traditional medical devices can be complex instruments, and it can’t be underestimated how the use of clear, precise labeling ensures that accurate information, directions, warnings, and risks are available to the healthcare professionals that use them.
However, digital technology is also changing the way healthcare is delivered and accessed with the use of wearable medical devices, giving patients empowerment, self-management, and personalization. The development and uptake of patient devices and wearables (such as vital sign monitors, blood glucose monitors, blood pressure monitors, pulse oximeters, and ECG monitors) is enormous, with the global market estimated at US $18.1 Billion in the year 2021 and projected to reach a revised size of US $38.9 Billion by 20262.²
The evolving UDI regulatory environment globally is forcing manufacturers to rethink their labeling processes. New regional UDI regulations have become a ‘tipping point’ in the medical device industry, and each new rule brings increased complexity and scrutiny.
Just a decade ago the original FDA UDI new rule was being introduced and phased in slowly, but since then, complying with countries and continents introducing their own systems, UDI requirements have become increasingly complicated. Each area has specifics of UDI implementation, including timelines and detailed requirements that can vary between jurisdictions. This should make UDI label management a core focus for medical device organizations.
Below highlights a few of the UDI systems either in force or coming into force, many of which are now aligning with International Medical Device Regulators Forum (IMDRF) standards:
In the U.S., The Food and Drug Administration’s (FDA) Unique Device Identification (UDI) system, must be used to identify medical devices sold in the U.S. from manufacturing right through distribution and to patient use. This rule requires all medical device labelers (the manufacturer, in most cases) to:
In the EU, the Medical Devices Regulation (EU MDR) and the In Vitro Diagnostic Regulation (IVDR) govern the production and distribution of medical devices. The EU MDR applies to medical devices that directly contact humans, while the IVDR applies to medical devices that perform an in vitro function. The EU MDR and IVDR were published in 2017 and then went into effect in 2021-22. Much like the FDA, these regulations are focused on improving patient safety and ensuring that patients have access to innovative medical devices
China’s National Medical Products Administration (NMPA) UDI was set up to regulate the establishment of unique device to strengthen the whole lifecycle management of medical devices. It applies medical devices sold and used within the territory of the People’s Republic of China. UDI refers to the code on any medical device itself or its package comprising figures, letters, or symbols, which is used for unique identification of medical devices.
India’s Central Drugs Standard Control Organization (CDSCO) has published requirements for a UDI system as part of its regulatory framework for medical devices. The new requirements include both a Device Identifier (DI) and Product Identifier (PI), and labels must include the UDI in both human-readable and machine-readable formats. Manufacturers must submit the DI portion of the UDI to a central database maintained by the CDSCO. India is aligning with global best practices and regulatory standards to facilitate international trade and ensure consistency.
Australia’s Therapeutic Goods Administration (TGA) is using a phased approach for implementing UDI over several years, with different timelines for different classes of devices. Higher-risk devices are prioritized for earlier compliance. Again, aligning with IMDRF international standards and submissions, it aims to enhance patient safety, streamline regulatory processes, and improve post-market surveillance by providing a standardized way to identify medical devices.
Often, labeling activities take a backseat during the product development cycle and labeling timelines can be underestimated. Logically, understanding requirements earlier in the product development would result in better time-to- market and reduced compliance risk.
Medical device labeling is difficult and is generally a “lagging” work stream for manufacturers, yet it is a leading cost driver due to recalls, corrections, and other problems. The manufacturer that successfully manages UDI and submission requirements for different markets can take the burden from internal teams and effectively support parallel design and engineering workflows.
Manufacturers do an excellent job of focusing on mechanical, electrical, and software engineering while labeling is more of an ‘unplanned journey’ that sits over the top of these processes and takes longer than expected. The reality is that labeling requires a deep integration of design controls and must be prioritized just like any other type of product data would be. Labels require a combination of both predefined and dynamic information and coordinating both often requires multiple integrated systems, including a PLM, an Enterprise Resource Planning (ERP), a Manufacturing Execution System (MES), and a Label Management System (LMS).
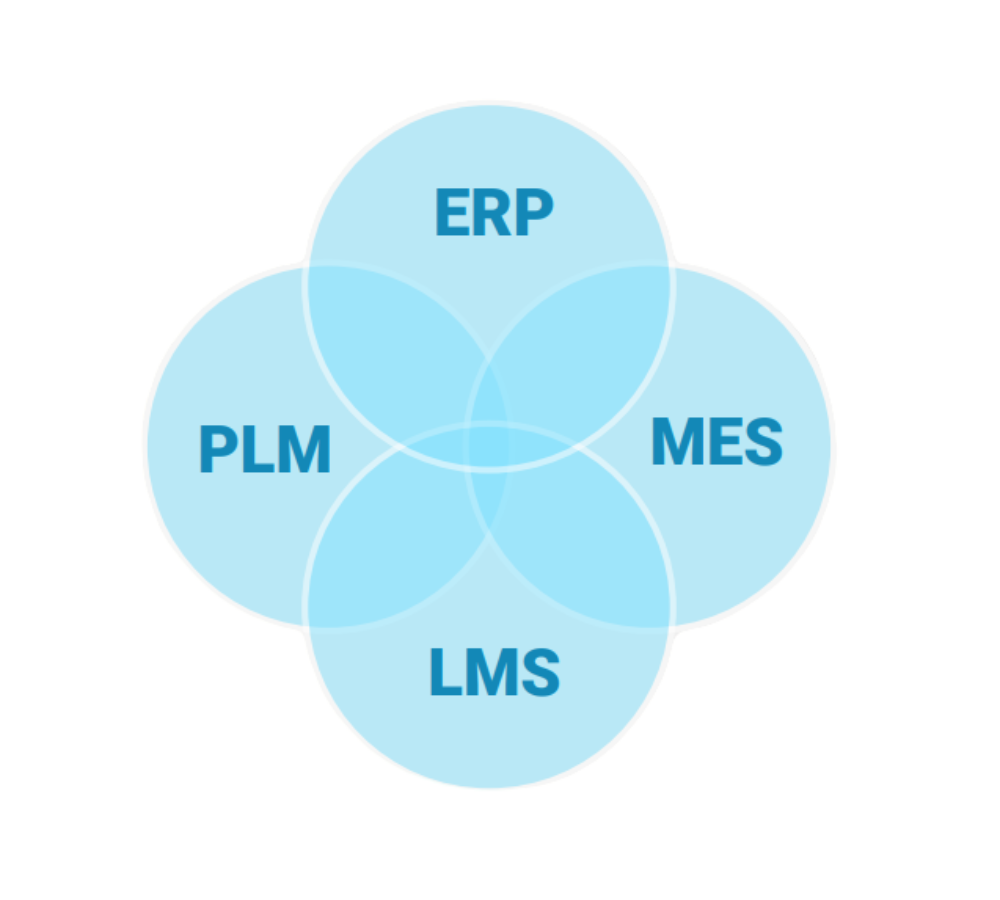
The label data journey starts with end user needs, which dictate what physically goes on the label. For the best effect, manufacturers must break that information down into the label objects that are under control; determine how to handle UDI records; and then feed those together into the label design.
The label data also must be verified, with all UDI information going on the label and being supplied to regulators for review. This is performed using health authority databases that are best aligned with early to ensure a successful, smooth UDI audit process. By sharing standardized data, cloud based labeling solutions help medical device manufacturers connect with business or trading partners, centralize the global labeling processes, and empower users across the organization.
Medical device organizations are always under pressure to speed up time-to-market to achieve profitability and growth goals and so must focus on labeling as a central part of their supply chain journey. But the answer is what’s beyond the label, the data, and its integrity. Data integrity mitigates risk and ensures quality, positively impacting across the medical device product lifecycle.
Labeling requires digital transformation. An end-to-end cloud-based labeling solution helps manufacturers get to market faster and helps minimize or eliminate manual data entry that is error prone and time consuming. This alone reduces the risk of mislabeling and improves time-to-market by removing antiquated manual processes.
Static labeling software that centers on graphic design tools cannot deliver these benefits, nor can it successfully manage traceability and compliance. For example, the SKU or trade item label data for medical devices and their packaging is likely defined in a PLM, along with other data about the product. When this data is defined, it becomes the master data set for the company’s products. This data is used to comply with UDI regulations, and it also facilitates and supports product brand management within the organization.
By integrating a cloud-based labeling solution into the supply chain journey, manufacturers can support UDI and meet the three challenges of traceability, patient safety, and UDI regulatory requirements.
An integrated cloud-based labeling approach addresses traceability complexities and supports overall lifecycle management while also transforming labeling compliance, accuracy, and efficiency for medical device organizations. The importance of the label becoming the ‘passport’ for the product journey must not be underestimated, as it carries the underlying data and enables complete traceability. Ultimately, distributed products will have a smoother transition across borders, limiting supply chain disruption and speeding up time-to-market.
Cloud-based labeling solutions ensure the correct label and data is used, translating into better patient care, and providing confidence that the right devices are always used. Labeling also helps employees properly rotate stock, monitor expiration dates, and keep inventory organized on a global basis.
Manufacturers are using cloud labeling solutions to help them navigate this increasingly complex regulatory labyrinth, where these solutions provide a single source of truth for all device data and ensure consistency and accuracy across all labels. This not only supports UDI compliance, but it also reduces the risk of errors that could result in mislabels, fines, recalls, and other negative outcomes.
By integrating cloud-based label management early in the design and engineering process, organizations can achieve ‘quality by design’ while also helping realize new efficiency and compliance benefits.
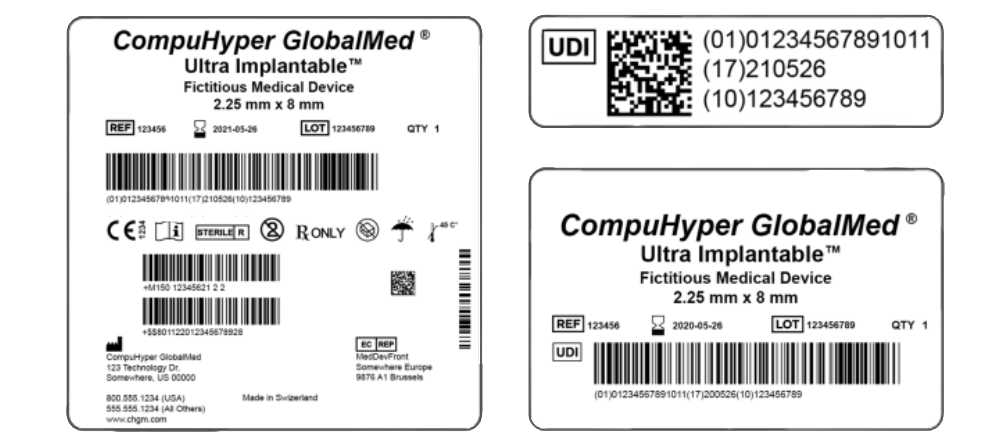
The new variances of UDI rules are now in effect for all, and their influence is expanding globally. UDI compliance isn’t just about having the right data set to meet compliance requirements; it is also about understanding the use of UDI from the regulator’s viewpoint. Put simply, regulators care about item tracking, health information, patient safety, and efficacy. Manufacturers, on the other hand, should be focused on data management that aligns with supporting industry standards, regulations, and efficiency within the supply chain.
In short, manufacturers must be compliant with many different regulations to ship medical products to multiple markets globally. If they can’t meet the local and regional labeling requirements, their products will not be shipped or used there.
Labeling excellence is a journey that touches all areas of a medical device product – from development, to manufacturing and production processes, and to distribution across the supply chain. It can be overwhelming as there are many considerations that go into building a label digitalization roadmap - including how and what to integrate, and how to ensure design quality while also addressing current and future regulations. When manufacturers have the right tool for the job, their users are more efficient, their pain points minimized, and errors avoided.
While labeling is just one part of manufacturing and distribution, the key is to look at the bigger supply chain journey when deciding which type of system to invest in. Manufacturers that give the discipline of labeling the effort and attention it deserves will ultimately navigate a smooth course to compliance and label quality while improving patient safety.
For further learning, watch our webinar on The challenge of labeling & UDI for medical devices.
